ACRC held a discussion to curb illegal rebates in the health and medical care industry on Dec. 1
- Date2017-12-26
- Hit1,868
December 21, 2017
ACRC held a discussion to curb the long-running illegal rebates in the health and medical care industry on December 1
The Anti-Corruption and Civil Rights Commission (ACRC, Chairperson Pak Un Jong) held a discussion on how to eradicate deeply entrenched illicit rebate culture in the medical and pharmaceutical industry on December 1stat the LW Convention Grand Ballroom in Seoul with the chairperson Pak Un Jong and various stakeholders from civic and medical groups, pharmaceuticals, and medical device companies being present.
Following the ACRC’s presentation on the theme of the discussion presided over by professor Lim Ji Bong from Sogang University, a number of relevant stakeholders from the Ministry of Health and Welfare, the Citizens’ Coalition for Economic Justice, the Korean Medical Association, the Korean Pharmaceutical Association, the Korean Medical Devices Industry Association, and the law firm Kim & Chang participated in the discussion to explore a variety of opinions regarding how to remove deeply entrenched practices that have long been customarily performed in the health and medical care industry.
While commonly recognizing that customary practices of giving or receiving pecuniary gains as rebates from pharmaceuticals in return for prescribing or purchasing their drugs cannot be sufficiently curbed by only beefing up supervision and punishment, participants pointed out during the discussion that a voluntary control system in which all relevant stakeholders engage should be strengthened.
In addition, it was discussed among participants that given that although providing rebates through third-party agencies including contract sales organizations (CSOs) has been problematic, there is a limit in reprimanding them since such third-party agencies are not specified as drug suppliers under the current Pharmaceutical Affairs Act, statutory grounds based on which to punish them should be more explicitly provided for in the Act with the system for filing a report on pharmaceutical contract sales being introduced.
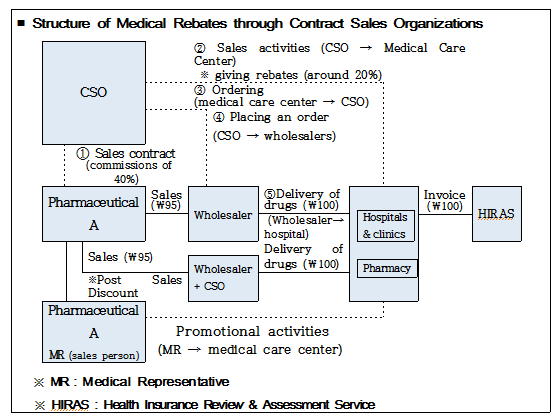
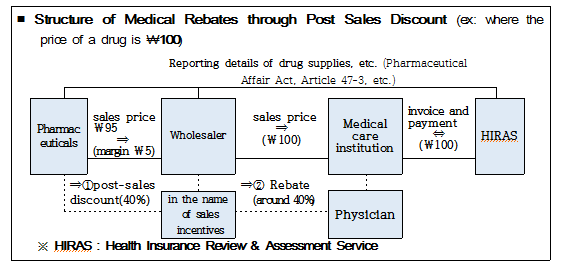
On top of this, participants also debated preparing a system of reporting on details of support provided on condition of sales to pharmaceutical wholesalers, etc. by pharmaceutical companies in a bid to prevent those companies from raising funds for illicit rebates through the method of post-sales discount, such as sales incentives or unit price discount, etc.
Regarding the comment on the possibility of abusing the support funds for an international academic conference held at home as a way of giving unlawful rebates since it is difficult to know whether such funds was administered properly or improperly, some suggested that conference’s preliminary organizational plan and a breakdown of expense use be made public on a web-site, etc.
The ACRC will establish institutional improvement measures to address customary practices of giving or receiving unlawful economic benefits known as medical rebates through consultation with relevant ministries and reflect various suggestions made during the discussion in the course of preparing such measures.
Attachment: [Annex]Measures to Eradicate Illegal Medical Rebates










